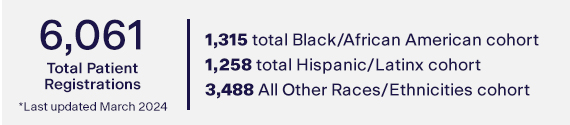
Note: Data reflects patient registrations. Total scans may not exceed cohort maximums defined by the study protocol.
Page last updated: April 30, 2024

Note: Data reflects patient registrations. Total scans may not exceed cohort maximums defined by the study protocol.
On behalf of the New IDEAS Study Leadership Team, we want to extend our sincere thanks to all the participants, caregivers, community partners, dementia practice and facility staff, and study stakeholders for your involvement in New IDEAS. Your participation and commitment to patient care and research has been instrumental in the Study’s accrual success.
A full list of dementia practices and PET imaging facilities who participated in New IDEAS can be found on our Site Finder.
We want to thank all participating dementia practices, clinic and imaging facility staff who have taken part in New IDEAS. Your efforts in recruiting a diverse group into this study are truly commendable. We appreciate your interest, time, and diligence in being a New IDEAS site and helping advance our mission of improved dementia care for everyone.
We want to extend our gratitude to the patients and caregivers of New IDEAS. Your partnership and participation in this study is invaluable to our team. Volunteers like you help advance science and medicine to improve care for those suffering from memory loss. Without your participation, this research would not be possible – thank you!
We want to express our gratitude for your partnership and support of the New IDEAS mission to address important gaps in knowledge that will help advance the study and care for diverse groups of people with dementia. Your expertise and dedication have been invaluable throughout this process.
While your involvement in this Study may be ending, we look forward to future opportunities for collaboration. Please go to https://www.alz.org/about to learn about opportunities for continued involvement in initiatives related to increasing access to memory care and education around Alzheimer’s and other dementias.
We are thankful for all the time and effort that our New IDEAS Study Champions spent to build the community engagement model for disseminating information about New IDEAS. Study Champions were trained volunteers who partnered with community organizations within their region to spread information about the New IDEAS Study.
Patients who participated in the study should ask their doctor about their own results. The New IDEAS Study team will share overall study results on the New IDEAS website when they are ready. Study results will also be made available at www.clinicaltrials.gov.
Study leadership is looking forward to sharing the Study results with the greater scientific community as well as offering opportunities for investigators to access Study data in the future to explore their own research questions, advance the science and celebrate inclusive research. Please check this webpage for ongoing updates on where to request data from New IDEAS when available for access. To request data from the IDEAS Study, complete the Data Access Request Application.
Read the most recent New IDEAS Study Newsletter here to learn more about ongoing initiatives. Read the most recent newsletter or past newsletters.
After careful consideration of numerous factors and evaluation of the risk-benefit tradeoff for patients, Study leadership has decided to end Study accrual on Friday, March 1, 2024 at 11:59pm EST. At that time, patient registration into the Study Portal will be closed. This deadline is applicable to all race/ethnicity cohorts of the Study. This decision was difficult; however, Study leadership is confident that this is the best course of action based on the following factors:
For more information, please read the full official Study Closure Memorandum and Study Closure Guide and Frequently Asked Questions for more information and next steps for your dementia practice or PET imaging facility.
Please contact the New IDEAS Operations Team at newideas@acr.org with any questions about the updates featuring on this page or past updates.